Theoretical Femtochemistry
Prof. Dr. R. de Vivie-Riedle, Department Chemie, LMU
The main focus of our research is the investigation of primary processes in photo-excited molecules and organic systems in their natural environment and their initial response to the incoming light field. Many of these processes proceed on the time scale of the nuclear motion, some even on the time scale of the electrons, which can vary from the sub- to the femtosecond range depending on the nuclear motion they are coupled to. Beyond mere observation we are interested in controlling these processes by modulated light fields. We develop and apply theoretical tools to solve the time dependent Schrödinger equation including laser interaction for molecular systems and design control fields to steer their motion. Depending on the size of the system we use multiscale methods combining parts that are described on the quantum level with parts that are described classically.
Electronic structure calculations for ground and excited states
Most of our work is based on the knowlegde of the potential energy surfaces (PES) and/or its gradients. Quantum chemistry methods are applied to calculate ground and excited-state PESs and to explore for critical features like minima, transition states and conical intersections. The latter are the key features for ultrafast (femtosecond) chemical processes. We apply these methods for molecules of chemical and biological interest, such as molecular motors, polyenes, sugar molecules, amino acids and RNA/DNA bases.
Quantum dynamics for nuclear and/or electronic motion in molecular systems
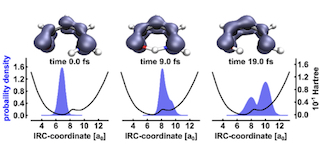 Pic. 1. Snapshots of the coupled electron-nuclear.
Pic. 1. Snapshots of the coupled electron-nuclear.
For the quantum part, we solve the time dependent Schrödinger equation for nuclear and electronic motion. We further develop our method for coupled nuclear- and electron-dynamics in molecules (NEMol) to higher dimensions, including pre-designed reactive nuclear coordinates. To explore the full dimensionality of the system we adapt the real time TDDFT formulation to propagate the electron density.
Quantum Control of molecular dynamics
We investigate the potential of few cycle pulses to enable directional control in molecular systems either by the control of vibrational wavepackets, electronic wavepackets or both. To unravel the mechanism we rely on our program code NEMol (developed in MAP) that handles nuclear and electron quantum dynamics on the same footing. We study molecular systems offering reachable conical intersections predestined for CEP control as predicted by us in 2012 [1].
A powerful method to derive optimal laser fields for nearly any physically-feasible target is Optimal Control Theory (OCT). Mostly OCT is used to design modulated light fields that are able to cooperate with the nuclear wave packet’s motion and to steer it, using multiple interference pathways. We will expand its application to ultrafast photoreactions involving many coupled nuclear and electronic degrees of freedom. Possible applications are proton transfer reactions (key reactions in life science) or molecular switches (devices for electron transfer, information processing and optical computing).
Quantum dynamics and control in complex environment
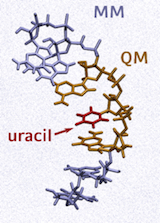 Pic. 2 The photostability of the
Pic. 2 The photostability of the
RNA base uracil investigated in
its natural environment.
New methods are developed to explicitly include the dynamic effects of the environment in the quantum dynamical treatment of the solute. [2,3] For the environment we are currently able to include typical solvents as well as RNA or DNA strands. This opens the possibility to intensify our activities in life science, helping to understand the physical, chemical and biological processes after strong field irradiation and to develop control fields preparing wavepackets that allow for new or improved spectroscopic experiments. [4]
Field-resolved spectroscopy
Field-resolved spectroscopy is a new technique to take IR fingerprints for very low concentration. Developed in the group of Ioachim Pupeza, integrated in the laboratories of Frence Krausz, first experiments show the possibility of applying this technique in cancer diagnostics. Blood samples of healthy and sick persons can be distinguished by tiny differences in the IR fingerprint region. To find out the possible origins of these tiny shifts we start with smaller model systems. We plan to simulate the induced molecular oscillating dipole for vibrationally excited molecules, like sugar molecules. For this project we can combine several methods, which we developed in the past years: coupled-electron nuclear dynamics, which now has to be extended to polyatomic systems, quantum dynamics and control in explicit environments.
We aim to simulate the free induction decay and its power spectral density for fructose in water. We exchange the environment to test their impact on the resulting signal. With our new tool – combined quantum classical propagation for system and environment- we will be able to detect spectral shifts and dephasing. Besides the clinical aspects, the high sensitivity of FRS opens the path to investigate fundamental physical aspects. Inhomogeneous line broadening due to environmental effects is omnipresent in liquid phase spectroscopy. Our theoretical chemistry group can decode this broadening by calculating the contribution of many single molecules in their individual environment. This situation mirrors the FRS experiments at extremely low concentrations.
Another major challenge at the application of FRS to clinical probes is the classification of the obtained data. The spectroscopic trace origins from many different poteins, which makes the assignment to either healthy or cancerous tissue highly complicated. Machine-learning techniques are planned to be used for this analysis, for example the adaptaion of the recently published Perseus platform (Nat Methods 13, 731 (2016)). With our new experience in machine learning [5] we plan to help with the analysis of FRS blood traces.
Representative references
- P. von den Hoff, M. Kowalewski, R. Siemering and R. de Vivie-Riedle, IEEE Journal of Selected Topics in Quantum Electronics 18 (2012) 119-129.
- S. Thallmair, J. P. P. Zauleck, and R. de Vivie-Riedle, J. Chem. Theory Comput. 11 (2015), 1987-1995.
- S. Thallmair, D. Keefer, F. Rott, and R. de Vivie-Riedle, J. Phys. B: At., Mol. Opt. Phys. 50 (2017) 082001.
- D. Keefer, S. Thallmair, S. Matsika, and R. de Vivie-Riedle, J. Am. Chem. Soc. 139 (2017) 5061-5066.
- J. P. P. Zauleck, and R. de Vivie-Riedle, J. Chem. Theory Comput. 14 (2018) 55-62.
