|
When a hydrogen molecular ion is
exposed to light, it breaks up into a hydrogen atom and a proton:
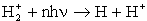
|
|
The kinetic energy of the fragments H and H+ is equal to the
difference between the photon energy
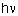 and the bounding energy of
a particular vibrational level. In our experiment we are able to distinguish
fragments originating from different vibrational levels (see
the experimental image). and the bounding energy of
a particular vibrational level. In our experiment we are able to distinguish
fragments originating from different vibrational levels (see
the experimental image).
At intensities higher than 1012 W/cm2 the coupling between
the ground 1sσg and the first
excited state 2pσu becomes very strong. In this regime
molecule-light system is usually described
by potential curves "dressed" with photons or with so-called light-induced
potential curves. |
 |
|
Potential energy curves of the two
lowest states of H2+ in a weak field. |
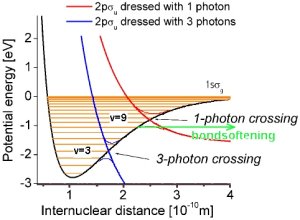 |
The main feature of these curves is the avoided crossing, a gap that opens up near the vibrational
level that is resonant with the laser light (v=9). The gap initiates a process that
is normally
forbidden - dissociation of molecular vibrational levels that lie below the resonant level. This effect is known as molecular bond-softening.
At again higher
intensities another gap opens due to three-photon absorption. Here a molecule
absorbs three photons and re-emits one photon, which leads to effective two-photon absorption. This effect is often called above-threshold dissociation,
because the molecule absorbes more photons than needed for its dissociation. |
|
Potential energy curves of the photon dressed states. |
|
When the intensity
becomes high enough, also ionization of H2+
may
occur:
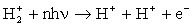
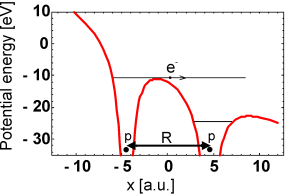
After the electron is stripped off the nuclei, the remaining
protons will repel due to
the Coulomb interaction acquiring kinetic energy of e2/(4πε0R),
where R is the separation between the nuclei at the moment of
ionization.
Already in early experiments, the measured proton energies turned out to be
much smaller than
it was expected. The explanation is that the ionization doesn't
occur at the equilibrium proton separation R0 of about 2 a.u., but at
a much larger R of approximately 5-12 a.u.
|
|
At intensities higher than 1012 W/cm2 the coupling between
the ground 1sσg and the first
excited state 2pσu becomes very strong. In this regime
molecule-light system is usually described
by potential curves "dressed" with photons or with so-called light-induced
potential curves. |
 |
|
Potential seen by the ionizing electron. |