|
|
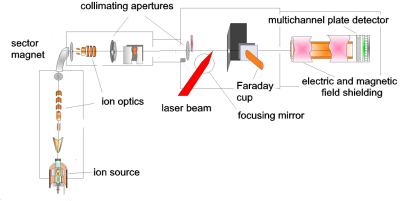
In our experiments, the molecular ions are
produced in an ion source by a DC electric discharge. They are
accelerated to kinetic energies of 11 keV, mass selected and then formed into a well
collimated ion beam (Figure 1).
|
|
|
Figure 1. The ion beam apparatus.
|
|
|
A
commercial femtosecond laser system produces up to 1000 laser pulses
per second, that are centered at a wavelength of 790 nm and have a pulse
duration below 100 fs.
The laser beam is focused by a lens onto the ion beam achieving very high intensities of up to 1015
W/cm2. This corresponds to electric field of about 109
V/cm. The fragments from the photodissociation and the Coulomb
explosion channel are detected on a multichannel plate detector. In this way, we
obtain a two-dimensional projection of the fragments' velocity distribution (Figure 2).
|
|
|
|
|
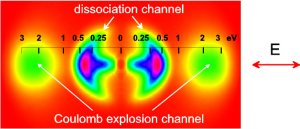
Figure
2. Velocity distribution of H2+
fragments as projected onto the two-dimensional detector (laser
intensity was I=1·1015
W/cm2 and pulse duration τ=90 fs). The direction of the laser polarization is shown with the
arrow. The
inserted scale marks kinetic energies per one fragment
in the polarization direction. Only one side of the image was
really
experimentally recoreded, the other side (obtained by
mirroring) is shown for completeness.
|
|
|
The fragments from the
dissociation (at lower kinetic energies) and the Coulomb
explosion (higher kinetic energies) are clearly separated.
The Coulomb explosion channel shows a much narrower angular
distribution. Also the fragments
with low energies in the dissociation channel have a narrow angular distribution
due to the nonlinear bond-softening effects. The narrowing of the angular
distribution with increasing intensity is shown in
Figure 3. |
|
 |
|
|
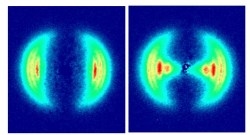
Figure
3. The two-dimensional projection on the detector of H atoms from the photodissociation of H2+ at two
different intrensities. The left image was made at an
intensity of I=3.5·1013 W/cm2 (τ=135 fs) and the right one at
an intensity of 1.5·1014 W/cm2 (τ=575 fs).
|
|
|
The
velocity distributions of fragments originating from different vibrational levels of
H2+ were distinguished here for the
first time. When intensity is
increased, the fragments from lower vibrational levels (v<9) can
dissociate via the bond-softening mechanism. They appear at lower
kinetic energies in Figure 3 (right) and particular in Figure 4, where the
data along the laser polarization direction are shown. |
|
 |
|
|
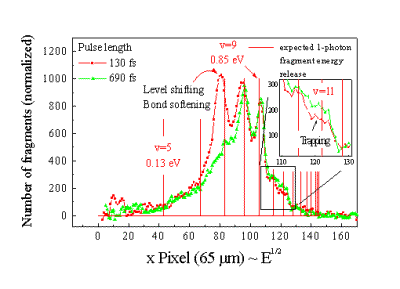
Figure
4. The projected velocity distribution of fragments
along the laser polarization axis. The pulse energy was 0.3 mJ
and the pulse durations were τ=130 fs (red) and τ=690 fs (green).
The comb marks the expected kinetic energy releases for the fragments
from different vibrational levels in one-photon dissociation
process. The trapping process for v=11 has been demonstrated
for the first time.
|
|
|
The
red curve measured at a higher intensity shows the appearance of
bond-softening fragments from vibrational level v=7 and v=8.
We have found that the energies of
these fragments are shifted to lower values with respect to
what is expected for an unperturbated molecule. This
level-shifting effect can also be understood as a classical
effect.
|