The hydrogen 1S-2S transition
Atomic hydrogen was crucial for the development of quantum mechanics because of
its simplicity. Whereas in the past the line spectrum in the solar corona or from
gas discharges was studied using prisms or grating spectrometers, narrowband lasers
whose frequency can be precisely tuned to one of the hydrogen lines have been
available for about fifty years now. In 2000 it became possible to measure the
frequency of the exciting laser directly with an atomic clock, the most accurate
physical measuring instrument. This was made possible by the Nobel Prize-winning
frequency comb
frequency comb technology.
The 1S-2S hydrogen lab in 2010
Suppressing the Doppler shift
Unfortunately, atomic hydrogen is very difficult to laser cool. In fact, spectroscopic
useful laser cooled hydrogen has never been achieved and all high-resolution experiments
thus far have been conducted on thermal beams. The main limitation of this method is
set by the Doppler effect. Even at cryogenic temperatures of 5K the mean thermal velocity
is still 300m/s because hydrogen is very light. A very efficient way to suppress the Doppler
shift is to use a transition such as the 1S-2S that requires two photons for its excitation.
With Doppler-free two-photon spectroscopy, the atoms absorb two photons from counter-propagating
laser beams. Although the frequency of the two laser beams appears shifted in the reference
frame of the atoms, these shifts have different signs and sum to zero.
There are two more important advantages of using this transition: Because there is no 1P
level, atoms in the 2S state cannot decay by emitting only one photon (dipole transition)
which makes the decay highly forbidden. The lifetime of the metastable 2S state is 0.12
seconds, which is about 8 orders of magnitude longer than the lifetime of the 2P level.
The resulting linewidth of only 1.3Hz makes the 1S-2S transition by far the best target
for precision measurements in atomic hydrogen. Last but not least, the two-photon transition
means that the required laser wavelength is 243nm instead of 121.5nm. While the longer
wavelength can easily be generated, the shorter so-called Lyman-alpha wavelength is close
to impossible, at least when it goes along with a narrow linewidth and sufficient power.
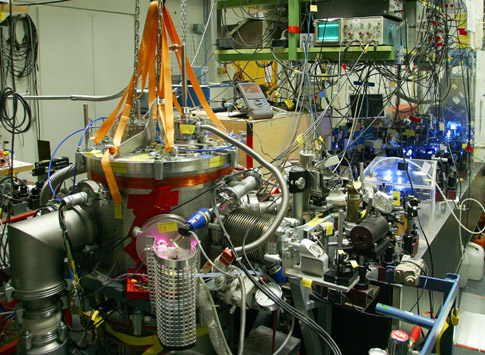
The hydrogen spectrometer
Molecular hydrogen is dissociated in a microwave discharge. The hot gas travels to the
5K cold copper nozzle where the atomic hydrogen thermalizes by collisions and escapes the
nozzle into the vacuum chamber. The hydrogen beam is defined by two apertures. With the help
of an enhancement cavity the power of the 243nm spectroscopy light is built up to increase
the intensity and to form a standing wave necessary for two photon spectroscopy. Atoms which
are excited before the excitation zone are subject to possible electric field shifts and are
therefore re-pumped to the ground state by a second, blue laser. In the excitation zone the
atoms are shielded against stray fields by a graphite coated Faraday cage. Atoms which are
excited to the 2S state are detected by quenching: The 2S state is so long lived that almost
no spontaneous emission from the excited atoms occurs. Therefore, we force the atoms to decay
by applying a small electric field. This mixes the 2S and the 2P state. The latter is
short-lived and decays by emission of a Lyman-alpha photon which we detect with a photo
multiplier tube (PMT).
The hydrogen spectrometer
Chopping the excitation light and waiting for a certain delay we can let the fastest atoms escape the detection region leaving only slow atoms contribution to the signal. In this way we can suppress the so-called second order Doppler effect. In contrast to the usual Doppler effect described above, the second order Doppler effect is based on a different physical mechanism. Special Relativity tells us that time flows at a different pace in a moving system. Hence, the transition frequency of the atoms appears slightly shifted. Even though this effect is tiny, our measurement accuracy on this transition of almost 15 digits requires its compensation.
Further Reading
C.G. Parthey et al.,
Improved Measurement of the Hydrogen 1S-2S Transition Frequency.
Phys. Rev. Lett. 107, 203001 (2011)
A. Matveev et al.,
Precision Measurement of the Hydrogen 1S-2S Frequency via a 920-km Fiber Link.
Phys. Rev. Lett. 110, 230801 (2013)