| Max-Planck-Institut für Quantenoptik (MPQ) | Laser Spectroscopy Group Home |
|
|
Overview |
Introduction |
Antihydrogen production |
Antihydrogen spectroscopy |
Experiment: 'CW-Lyman-alpha' |
In this manner the 1S-2S transition in hydrogen has been measured in our group to an unprecedented accuracy. We intend the use the same techniques for antihydrogen. There is, however, one difference: we don't have as many antihydrogen atoms yet as used for the hydrogen experiment but only a few thousand. That is more than 10 000 000 000 times less than in typical hydrogen experiments! Obviously we will have to be very careful with our precious atoms, and find ways to measure the 1S-2S transition with good efficiency and accuracy. For this reason we aim to accomplish at least the following two points:
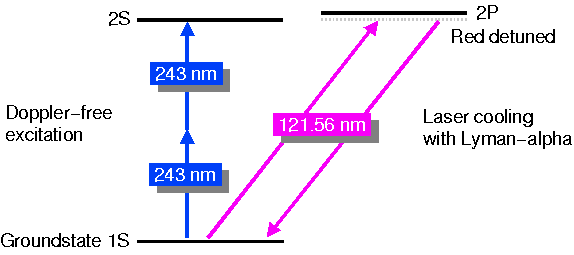
Laser cooling with Lyman-alpha light
as depicted in the picture below serves several purposes. The 'hotter'
the antihydrogen atoms are, the larger the volume they occupy in a magnetic
trap. For two-photon 1S-2S spectroscopy this is a big problem as the 243
nm light has to be focused to a small spot to increase the transition probability
(scales with the square of the laser power density). If the atoms are moving
wildly around in a large volume, then most of the time they are not in
the 243 nm light to excite the 1S-2S. If we, however, laser cool the atoms,
they collect in a much smaller volume of the trap at the potential minimum,
and we have a bigger chance to hit them with 243 nm light. Another effect
of the cooling is that the atoms experience a smaller range of magnetic
field. This reduces the line width and systematic error introduced by the
magnetic field dependence of the 1S-2S transition (due to a small relativistic
effect). Also very import is that due to the lower temperature the second-order
Doppler effect is reduced.
 |
|
|
Laser cooling antihydrogen
To cool atoms down one has to reduce the speed at which atoms move around. This speed is normally quite high. Antihydrogen at room temperature has a speed of a few thousand meters per second. To change the atom's velocity we can make use of the fact that absorption of a photon imparts a little bit of momentum equal to p = h / lambda, where h is Planck's constant and lambda is the wavelength of the light that is absorbed. The velocity change per absorbed photon is typically between a few cm up to a few meters per second. To slow atoms down (instead of accelerating them) we have to make sure that only atoms that come towards the light source absorb photons. This can be done using the Doppler effect. When an atom moves towards the light source it sees the photons at a shorter wavelength than when it is standing still. Of course an atom moving away will see a higher wavelength. If the cooling laser is detuned to a little bit too long wavelength (red detuned) for the atomic transition on which the cooling takes place, than only atoms moving towards it will come in resonance and absorb photons. After each absorbed photon the atom is in an excited state (the 2P in the case of antihydrogen). Before absorbing the next photon the atoms first has to release the absorbed energy and momentum again. This is done by fluorescence which (when no external fields are present) has no preference for any certain direction. As a result the momentum kicks by the fluorescence photons average out to zero over many absorption-fluorescence cycles. The trick of laser cooling is therefore that the absorption of photons is always against the direction of motion, slowing the atom down, but the fluorescence averages out to zero. If you take six laser beams coming from all sides you can cool the atoms in all directions. Such a configuration is called a 'molasses'.
Laser cooling is normally done on transitions in the infrared
(e.g. in rubidium) or visible wavelengths (e.g. sodium). For (anti)hydrogen
you need 121.56 nm to induce the 1S-2P transition. This short wavelength
results in a photon momentum almost an order of magnitude larger than e.g.
for rubidium laser cooling. At the same time, (anti-)hydrogen is the lightest
atom that exists, so that the laser cooling effect (velocity change of
3 m/s per absorbed photon) is very strong indeed. That is the good part
of it. It also has a down side. How low in temperature you can go depends
on two things in this simple laser cooling scheme. In a steady state when
the atoms are as cold as they can get, photons are emitted and absorbed
in all directions. This results in a random minimum motion depending on
the mass of the atom and the momentum of the photons. Due to the light
antihydrogen and short wavelength photons the minimum velocity is roughly
a few meters per second, which is two orders of magnitude larger that for
alkali atoms. The second, and stronger limitation, is due to the line width
of the 1S-2P transition. A large width reduces the selectivity you have
to make sure that only atoms coming towards the 121 nm light source will
absorb light. As the line width of the 1S-2P transition is 100 MHz, this
results in a minimum temperature you can get of ~ 3 mK.
For 1S-2S spectroscopy a temperature of 3 mK is very
nice. If we want to do experiments with gravitation, we would like/need
to have colder antihydrogen. How to do this is one of the many questions
that still have to be solved.
Laser cooling antihydrogen in a magnetic trap
The intensities of 121.56 nm available right now
do not allow laser cooling of free flying atoms. The interaction time would
be to short and the atoms will simply fly on to annihilate with the walls
of the experiment. High resolution spectroscopy and laser cooling therefore
probably requires that the atoms are somehow trapped for longer periods
of time in the same volume. Without going into detail here, this can be
done by the tiny force that a magnetic field excerts on a magnetic moment
an atom can have. The 1S ground state of (anti)hydrogen actually consists
of four states with different magnetic moments. Two of them lead to a potential
minimum for the atom in a magnetic field minimum. If those atoms are cold
enough they can be trapped in this potential minimum. The other two states
see a potential maximum in the magnetic field minimum, and are therefore
quickly accelerated outwards and lost. One of the tricky thing of laser
cooling antihydrogen in a magnetic trap will be to keep them in the right
(trapped) magnetic substrate. The atoms can end up in the non-trapped state
by fluorescence from the 2S (upper state for two-photon spectroscopy),
or by fluorescence from the wrong (3 out of 4) magnetic substates of the
2P state (the laser cooling).
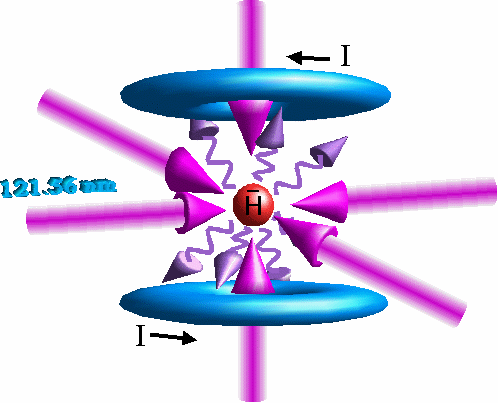
Schematic of laser cooling antihydrogen in a quadrupole
magnetic trap (magnetic field is zero in the center,
and increases in all directions)
Three remarks to this picture above: